Lei Fengwang press: Wang Tianmiao, Professor, doctoral tutor, Cheung Kong Scholar of the Ministry of Education, Professor, National Robotics Expert Team Leader of National 863 Program, Honorary Director of Robot Research Institute of Beijing University of Aeronautics and Astronautics. The main research direction is advanced robot technology, and outstanding achievements have been made in medical robots, bionic robotic fish, and embedded technology.

According to the 2016-2020 China Medical Robot Industry In-depth Survey and Investment Prospects Forecast Report (Source: CIC Advisor), the global medical device market will reach US$514 billion in 2020, and the compound annual growth rate will be 2013-2020. 5%. In 2020, the global medical device R&D investment will reach 30.5 billion U.S. dollars, and the compound annual growth rate between 2013 and 2020 will be about 4.2%. The final direction of medical devices must be intelligent in the future, and continuous investment in research and development will also move in this direction. Medical robots are the ultimate direction of intelligent medical devices, and their proportion of the medical device market will increase. The North American market is still the largest market, and the future focus will gradually shift to the Asian market.
At the CCF·GAIR Global Artificial Intelligence and Robotics Summit on August 13, 2016, Professor Wang Tianmiao gave a keynote speech entitled “Thinking about the Development of Robots—Academic Industry to Industryâ€. In the gap before the speech, Professor Wang Tianmiao shared the topic of medical robots that people care about.
Academia to industryLei Feng Network (search "Lei Feng Net" public concern) : From the academic world to the industry, where do you think the robot will be the first to generate an upsurge?
Wang Tianmiao: The increase in manpower costs and the increase in the cost of rental land may be the main reason for companies to use robots. At present, high-end robots in China are mainly imported. Low-end manufacturing, grinding, injection molding, and handling of industrial robots are becoming more and more localized. This is the current state of development. I believe that in the future, robots will first emerge, including robotic assistants, chat robots, and companion robots, and will appear in banks, families, hotels, and hospitals. I believe these points will be confirmed in the next five years.
The development bottleneck of medical robotsLei Feng network: As you all know, you have a lot of achievements in the field of medical robots. Would you like to ask if there is any bottleneck in the process of the transformation of the current medical robot from the academic world to the industry?
Wang Tianmiao: The important point of differentiating medical robots from other robots is that they involve life and health and must ensure safety. Therefore, they must be maintained through very strict means such as qualification certification and clinical trials. However, the corresponding experimental period is long, and the slow progress of research and development also restricts the development of medical robots.
Differences in the research and development of medical robots between academic and practicalLei Fengwang: In view of these development bottlenecks, what are the different ways of research and development in academic research and industrial practice?
Wang Tianmiao: Academically, we generally proceed from a complete system, establish academic plans, set targets, and conduct safety controls. In practice, it may not be able to be fully rolled out yet. It must focus on a certain aspect, such as orthopedics, paediatrics, and so on.
What are the mature medical robots in the industry?Wang Tianmiao: "At present, in the world, first of all, the United States Da Vinci surgical robots do better, and then Israel's Rewalk is more prominent in the wearable field, and France's Rosa robot is also widely used. From the domestic point of view, like Tianzhi Hang, which has focused on orthopedics, has been licensed and listed on the New Third Board. The products have been used in Jishuitan Hospital and other domestic hospitals."
Da Vinci Surgical Robot
The Da Vinci robot is currently the most advanced robotic surgical assistant system. It was first developed by Intuitive Surgical Inc. and is an outstanding representative of a new generation of minimally invasive surgical techniques. With the aid of intelligent robotic arm aids and high-definition 3D imaging systems, it integrates many new disciplines and realizes minimally invasive surgery, functionalization, intelligence and digitization. It is designed to perform complex surgery using minimally invasive methods. The "surgical robot" consists of three parts. The following figures from left to right are: surgeon's console, bedside robotic system, and imaging system.
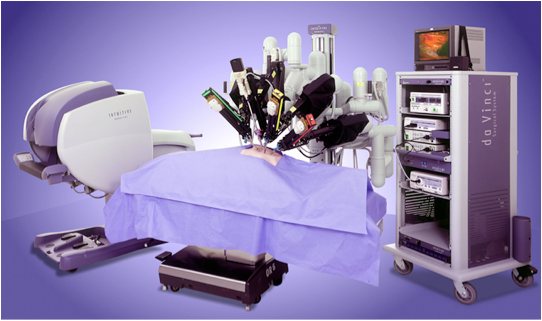
DaVinci Surgical Robot System
Rewalk
The Rewalk Robotics, which is designed and manufactured by Rewalk Robotics, a well-known power exoskeleton system manufacturer in Israel, has been using the concept of restoring human walking ability and accelerating physical rehabilitation. The product line includes ReWalk Personal's personal exoskeleton and clinical version of Rewalk Rehablitation. Its main purpose is to assist the lower limbs. The paralyzed patient can stand and walk again.
ReWalk Personal 6.0, Rewalk Robotics's sixth-generation personal version, is based on its predecessor and is also lighter and faster. It is designed for use in home, work, or social settings, through sensors and monitors. Keep the patient standing, walking and climbing stairs.
This product uses a lighter plastic material and changes the piggyback battery compartment to the hip side to reduce weight and increase wearing comfort. And, support private custom, so that users can also adjust the size and shape of the exoskeleton according to their height and size. It is easier to wear and it is only necessary to bind the exoskeleton to the legs, but it is necessary to control the remote control with both hands to use it. The built-in gravity sensor can automatically determine the center of gravity of ReWalk 6.0, so that the stepper motor produces the correct steering, and the optimized algorithm makes walking more natural.

ReWalk Personal 6.0
Rosa
Established in 2002 and headquartered in Montpellier, South France, Medtech SA is a global leader in the development of surgically assisted robot systems. The company's flagship ROSA brain robot has been approved by Europe, the United States, China, Canada and Australia. Medtech SA has installed 51 surgical systems worldwide and is located in major neurosurgical centers worldwide, including the Cleveland Clinic and the Massachusetts General Hospital. At present, ROSA robotic surgical systems have been installed in four of China's top neurosurgical hospitals.
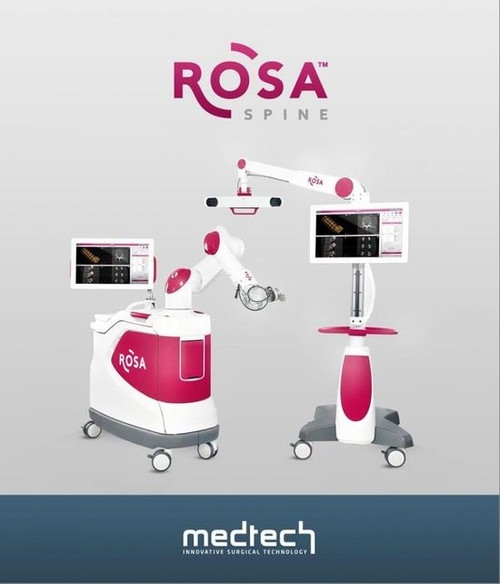
ROSA robot
Tian Zhi Hang
Tianzhihang is mainly in the field of orthopedics. Orthopedic surgery does not involve internal organs. It is easier to model and is suitable for robots. The company has been listed on the New Third Board.
Surgical robots belong to the third category of medical devices. Clinical verification is required to obtain a license. The time for obtaining the license is 2-5 years. Tianzhihang products originated from the results of the 863 project completed by Beijing University of Aeronautics and Astronautics and Beijing Jishuitan Hospital. Tian Zhihang obtained the registration license for orthopedic robot products in February 2010. This is China's first medical robot product with completely independent intellectual property rights. The company has thus become the fifth company in the world to obtain a registration license for medical robots.
Currently in Beijing Jishuitan Hospital, 301 Hospital, No. 3 Hospital of Hebei Medical University, No. 2 People's Hospital of Shenzhen, No. 4 People's Hospital of Zigong City, Haidian Hospital of Beijing, Changping District Hospital of Beijing, Karamay Central Hospital of Xinjiang, Hebi City People's Hospital and other medical institutions have been applied.
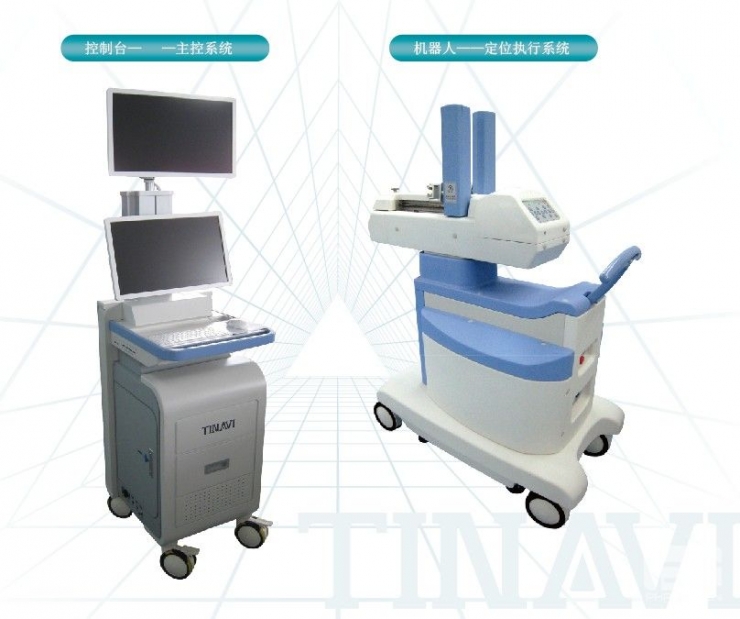
Tianzhihang Orthopaedic Robot Navigation and Positioning System (GD-2000)
Jiangmen Ever-smart Intelligent Control Instrument Co., LTD , https://www.fluhandy.com