Gas sensors for the detection of flammable, flammable and toxic gases, and/or oxygen consumption. This type of device is also widely used in industry or fire fighting. Various materials such as inorganic semiconductors, conjugated polymers and carbon nanomaterials have been explored for the manufacture of gas sensors.
Among them, graphene-based gas sensors have recently attracted strong attention. As a sensing material for gas sensors, the excellent properties of graphene are unique and attractive.
First, graphene has a large theoretical specific surface area (2630 M2G ≤ 1). All atoms of a single-layer graphene sheet can be thought of as surface atoms and molecules that they can adsorb gas, providing a maximum sensing area per unit volume. Second, the interaction and adsorption between graphene sheets may be due to weak van der Waals forces, with strong covalent bonds. The perturbation of all these interactions will be the electronic system of graphene, which can be easily MONI-tored by convenient electronic methods. Third, graphene charge carriers have a static mass of zero near their Dirac point and graphene exhibits significant high carrier mobility at room temperature, making graphene conductive over silver and having room temperature Among the substances is the lowest resistivity.

In addition, graphene has inherently low electrical noise, and its high quality lattice, along with its two-dimensional structure, enables it to shield more charge fluctuations than one-dimensional. As a result, a small amount of additional electrons can cause a significant change in the conductivity of the graphite. The resistance of a gas-adsorbed graphene sheet caused by a very small change is even reduced to a molecular level that is detectable. Moreover, graphene sheets can also be used to fabricate four-point devices to effectively eliminate the effects of contact resistance. Chemically converted graphene can also be synthesized at relatively low cost on a large scale. In fact, graphene materials have been widely used to detect toxic and explosive gases.
Graphene structure

As shown in the figure, graphene is a planar film composed of a carbon atom and a sp2 hybrid orbital to form a hexagonal honeycomb crystal lattice, and has a two-dimensional material having a carbon atom thickness.
Characteristics of graphene
After the graphene adsorbs the target gas, its conductivity changes. By determining the relationship between the conductivity change and the gas concentration of the target table, the concentration of the target gas can be measured by measuring the change in the conductivity of the graphene. It belongs to a resistive sensor.
The detection of the gas through the graphene material is mainly based on the change in conductance of the sensing substance. Gaseous adsorbates have different compositions and structures that interact with graphene in different modes. Inert closed-cell adsorption like water does not induce graphene to detect localized distortion states, which affect the conductance of graphene by shaking the electrons within the graphene sheet or between the graphene and its substrate. On the other hand, open cell adsorbates such as NO 2, alkali metals and halogens are chemically active; they can act as temporary dopants that contribute to electrons or holes to graphite and change their electron concentration. These molecules bind to the ions of graphene but weakly hybridize to the graphene bands. Another adsorbate is covalent bond adsorption, including H and OH radicals, which form covalent bonds with graphene.
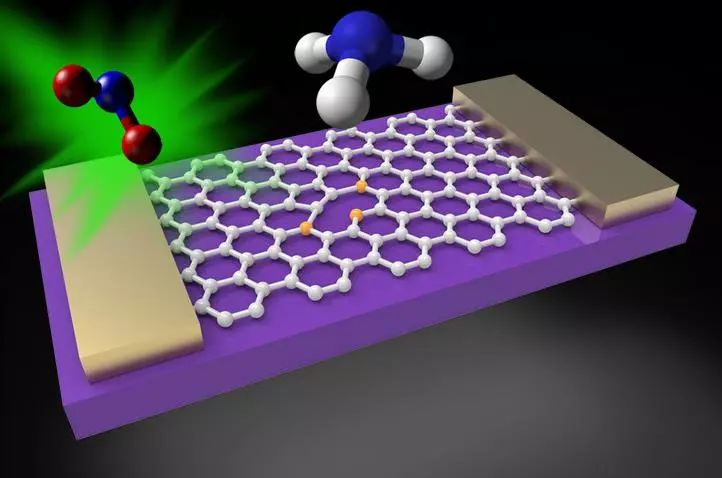
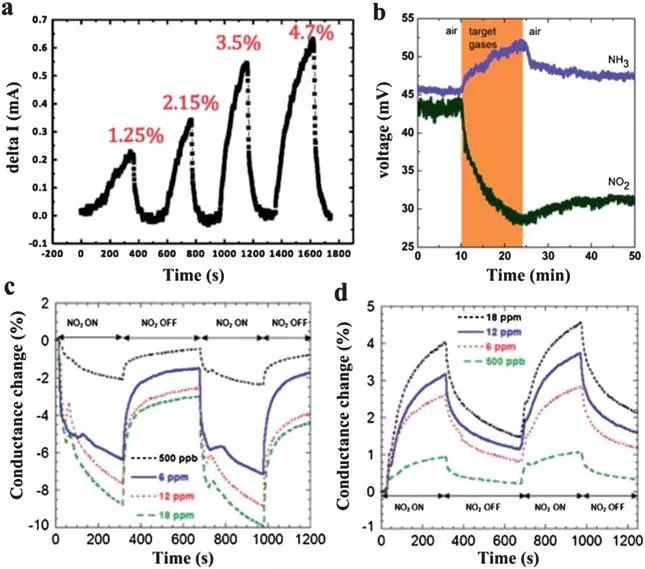
Graphene is essentially a pn-type semiconductor. When it is exposed to various gases, the direction of its conductance response may be different. Adsorption of electron-attracting gas molecules such as NO2 enhances the doping level of graphene and increases its conductance. On the other hand, an electron donor such as NH3 decomposes the graphene of the stock solution and lowers its electrical conductivity.
Various graphene composites have also been used as sensing materials to improve the performance of graphene-based gas sensors. Among them, graphene/polymer composites generally have a porous microstructure to accelerate gas diffusion in the sensing layer. In this case, the two components of the composite can adsorb gas molecules, facilitating the conductance change of the sensing layer. Nanoparticles of noble metals such as Pt and Pd have been immobilized on graphene sheets to catalyze the reaction of gases in order to increase the sensing signal. Oxygen or water molecules on the surface of the adsorbed graphene and its composites can also interact with the sensing molecules and contribute to the sensing response. In particular, for graphite/metal oxide composites, oxygen adsorption is sometimes critical to achieve detection reactions. The absorbed oxygen molecules are trapped from the metal oxides and may be converted to ionic species. After introduction of the detected gas species, the concentration of electrons on the surface of the metal oxide changes because of the interaction between the gas and the adsorbed oxygen ions and causes a change in the conductance of the sensing layer.
Structure and configuration of graphene gas sensorChemical resistance is the most widely used configuration of gas sensors. In this case, the gaseous analyte is detected by measuring the change in resistance of the induced adsorbed gas molecular sensing layer. The advantages of this type of sensor are its simple fabrication and direct measurement. The figure below shows the structure of a four-point resistor interdigital gas sensor. A tiny sized heating plate is introduced into the device to control the sensed temperature. The sensor can be used to detect NO 2 , NH 3 , dinitrotoluene (DNT), and the performance of the sensor is strongly dependent on temperature.
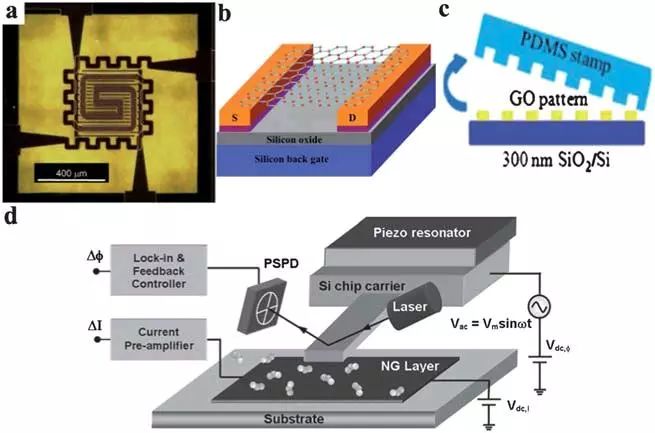
Field effect transistors (FETs) are also used to sense gases. In this case, the drain current of the FET depends on the gate bias, and it can be effectively changed by exposure to the target gas.
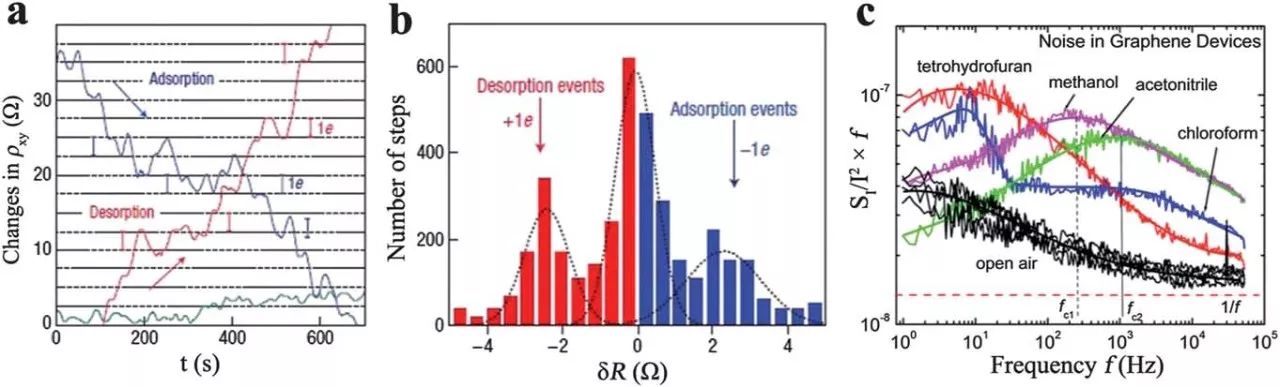
The performance of a FET sensor is strongly dependent on the on/off current ratio of the device. Higher on/off ratios can usually be used for higher sensitivity. Several methods have been used to create energy gaps in graphene sheets to achieve on/off ratios of field effect transistor devices, including conventional nanolithographic patterning, synthesis of graphene nanoribbons, and separation of fine graphene sheets from bulk graphite. . The charge carriers in graphene sheets are bipolar due to their unique atomic thick two-dimensional structure, and the charge density can be continuously adjusted by application in the field of view. These characteristics make graphene advantageous for the fabrication of field effect transistors. In this sensor, the suspension network of RGO platelets acts as a conductive channel by bridging the source and drain electrodes. When the NO 2 molecule is adsorbed onto the surface of the graphene sheet, the local carrier concentration is increased, and the signal can be monitored by a device such as a transistor. Electrons or holes can be used as the main charge carriers in the graphene field effect transistor depending on the value of the gate potential (VG).
Surface acoustic wave (SAW) technology has also been studied for gas detection. Gas sensing by this type of sensor is a function of the change in mass caused by the change in mass and/or the change in conductance of the sensing layer exposed to the gas molecules. Detection of CO and H2 by SAW sensors. The sensing response was measured to be about 1.7 or to 1% H2 or 1000 ppm CO. Although the reducing properties of the two gases are 7.0 Hz, the direction of the frequency shift is different.
This phenomenon can be explained as follows. A SAW sensor responds to both mass and surface conductance changes. However, the molecular weight of CO is 14 times higher than that of H 2 . Therefore, the main factor in the reaction of carbon monoxide is the change in mass, while the reaction of H 2 is mainly due to the conductance change of graphene.
Today, most graphene-based gas sensors have a thin layer structure. A single raw or CVD graphene sheet can be transferred to a rigid or flexible substrate to form a sensing layer. The metal electrode is then deposited on the surface of the graphene with a shadow mask to construct the final sensing device. Thin layers of CCGS can be made from their suspension by drop casting, spin coating, spray coating or dip coating. Bulk graphene materials have also been used to fabricate gas sensing devices. For example, graphene foam has a continuous three-dimensional web, and a CVD method and a nickel foam are prepared for use as a template. These foams have a large porosity and gas molecules can easily diffuse to the surface of the inner graphite wall to aid in sensing the signal.
Development and technical types of graphene gas sensorsOriginal graphene gas sensor
In 2004, Heim and colleagues prepared high-quality single-layer graphene by mechanical stripping. They stripped the fragments of graphite and tape to separate single-layer graphene sheets with a nearly ideal crystal structure. In 2007, mechanically stripped graphene used by Novoselov et al. was used to detect gases.
After the above groundbreaking work, several other teams also studied the experimental performance and theory of the original graphene, and their sensors are capable of detecting a variety of gases, such as NO 2, NH 3 , CO 2 , etc. The performance of these sensors can be determined by several factors such as temperature, the rate of gas flowing through the object, and the ratio of the length to the width of the graphene sheets. The report is based on the original single-layer graphite, which can selectively detect the vapor of different chemicals.
Most of the above graphene-based gas sensors have poor reversibility, similar to carbon nanotube-based devices. Thermal energy is often insufficient to overcome the activation energy for desorption. Insufficient recovery of the sensor will result in unreliable sensing output along with low sensitivity. To solve this problem, ultraviolet (UV) light can be used in the process of gas detection to clean the sensing layer. Under the ultraviolet light, the sensing devices at these two ends can achieve ultra-high sensitivity. The LOD of NO is tested to as low as 158 PPQ, and this value is about 3 times lower than under the same conditions as a CNT-based sensor. In addition, the sensor also shows high detection limits on other gases, including NH 3 , NO 2 and NO, in the range of 38.8 to 136 percent.
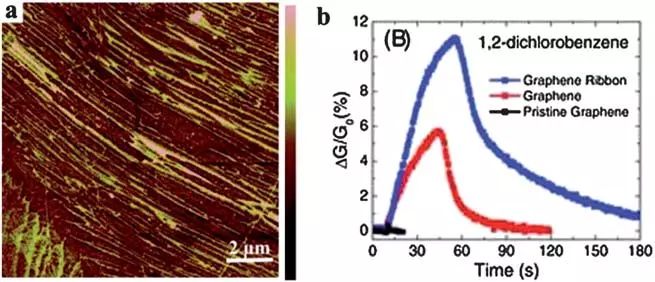
Epitaxial growth is another method for the preparation of large-area single or multi-layer graphene. When the SiC substrate is heated under ultra-high vacuum, the silicon atoms sublime from the substrate and carbon atoms rearranged into a graphene layer. The thickness of the graphene layer depends on the annealing time and temperature. This method can directly grow graphene sheets on a SiC substrate; thereby avoiding the transfer process before the device is fabricated. The performance of the graphene-based gas sensor adjusted in the selection range of the substrate plays an important role.
Graphene/Polymer Composite Gas Sensor
In the study of the sensing properties of graphene, it has been found that conventional lithography is inevitably on the surface of graphene behind polymer photoresist (eg, polymethyl methacrylate, PMMA). . This polymer residue is chemically doped with graphene and enhanced carrier scattering and also acts as an adsorbent layer on the surface of the graphene that concentrates the analyte molecules.
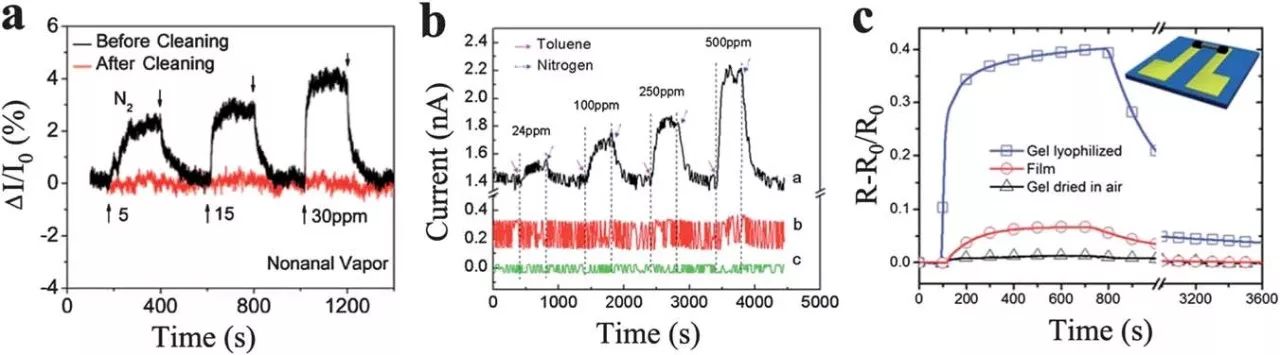
The graphene sensor and the polymethyl methacrylate residue exhibited a strong electrical reaction in the ppm-grade furfural gas phase. Furthermore, the reaction is reversible and the signal recovery time is short. However, after removal of the polymer residue, the response is drastically reduced. GO/Poly (GO/PPR) organic vapor detection composite sensor. The composite sensor exhibits excellent performance in the selective detection of toluene, with a fast, linear and reversible reaction with a high sensitivity of 9.87 to 4 ppm ≤ 1 (DG/G0). We attribute this to the unique microstructure of the sensor's highly sensitive GO/PPR composite membrane.
The addition of GO increases the mechanical properties of PPR and results in continuous porous composite lumens, which leads to the formation of uninterrupted conduction paths. The PPR layer GO flakes can adsorb toluene vapor and increase the conductance of the composite lumens. We also produced a composite aerogel NH3 sensor prepared from the corresponding hydrogel according to GO/polypyrrole (GO/polypyrrole). Polypyrrole aerogels are expected to have a wide range of gas sensing applications due to their large specific surface area and high electrical conductivity. However, their precursors (such as polypyrrole hydrogels) cannot be easily obtained because of the insolubility of polypyrrole. In this case, a GO/polypyrrole composite hydrogel is produced by in-situ polymerization of a pyrrole monomer in an aqueous dispersion of GO, which in turn is freeze-dried into an aerogel.
As shown in the figure, the resistance of the chemical resistance based on GO/polypyrrole aerogel is increased by 40% in the range of exposure to 800 ppm of NH 3 for 600 seconds, and the value is based on pure polypyrrole lumens (7%). It is much higher. This increase in resistance is believed to be associated with the dedoped NH3 pair of polypyrrole. The high performance of this sensor is partly due to the ultra-thin polypyrrole layer of the composite, which is more critical, and the aerogel with large pores is also critical.
Graphene/metal or metal oxide composite gas sensor
One-dimensional nanostructures of metal oxides such as zinc oxide, tin dioxide, cuprous oxide nanowires (NWS) or nanorods (NRS) have been widely explored for sensing applications, mainly due to their large specific surface area and high length. Wide ratio and excellent mechanical flexibility. However, the low conductivity of these nanostructures often limits their performance. Mixing them with two-dimensional graphene sheets to form a hybrid architecture can improve their sensed behavior.
Kohl et al. developed a zinc oxide/graphene mixture obtained by growing vertically aligned ZnO nanorods (zinc oxide nanorods) on a CCG film to detect H 2 S at room temperature. In this case, adsorption on the surface of the oxygen-oxidized zinc nanorods is critical to achieve excellent sensing performance, which may be due to the fact that the adsorbed oxygen is converted into an ionic species by the capture of electrons from the zinc oxide. Therefore, the sensor exhibits an environment of increased oxygen resistance. The electron density on the surface of the ZnO nanorods after the introduction of hydrogen sulfide is increased due to the interaction between H 2 S and the adsorbed oxygen ions. Therefore, the resistance of the zinc oxide NR/graphite composite based sensor is reduced.
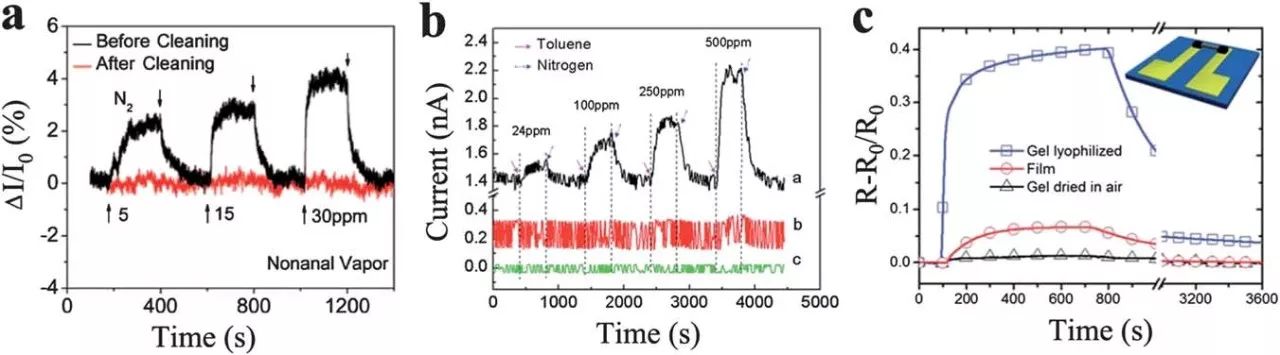
The use of a CVD-graphene sheet along the thin metal layer as a vertically oriented ZnO nanorod (zinc oxide nanorod-GR/M) (Fig. 9a) with the top electrode 0.119 mixed architecture can maintain sufficient between the nature reserves Space is used to maximize the contact of the surface area with the target gas, allowing for fast and easy gas transport. In addition, the flexible metal foil zinc oxide/graphene blend can accommodate repeated bending, straightening, no mechanical failure, and those with higher transmission visible light on the glass substrate.
The response based on a hybrid sensor exhibits 9 or 90 is 10 or 50 ppm of ethanol. The uniform distribution of metal oxide nanorods can significantly affect the sensing performance of gas sensors based on their composites with graphene. For example, a three-dimensional framework of tin dioxide/graphite with 3 different morphology and Ord-like SnO 2 nanostructures has been used to fabricate gas sensors. Wherein, the composite lumen has the highest sensitivity of hydrogen sulfide detection by the nanorods having a diameter of about 50 nm and a number density Ï2 of 285 mm. In contrast, pure tin dioxide flower-free graphene-based sheets exhibit relatively weak signals.
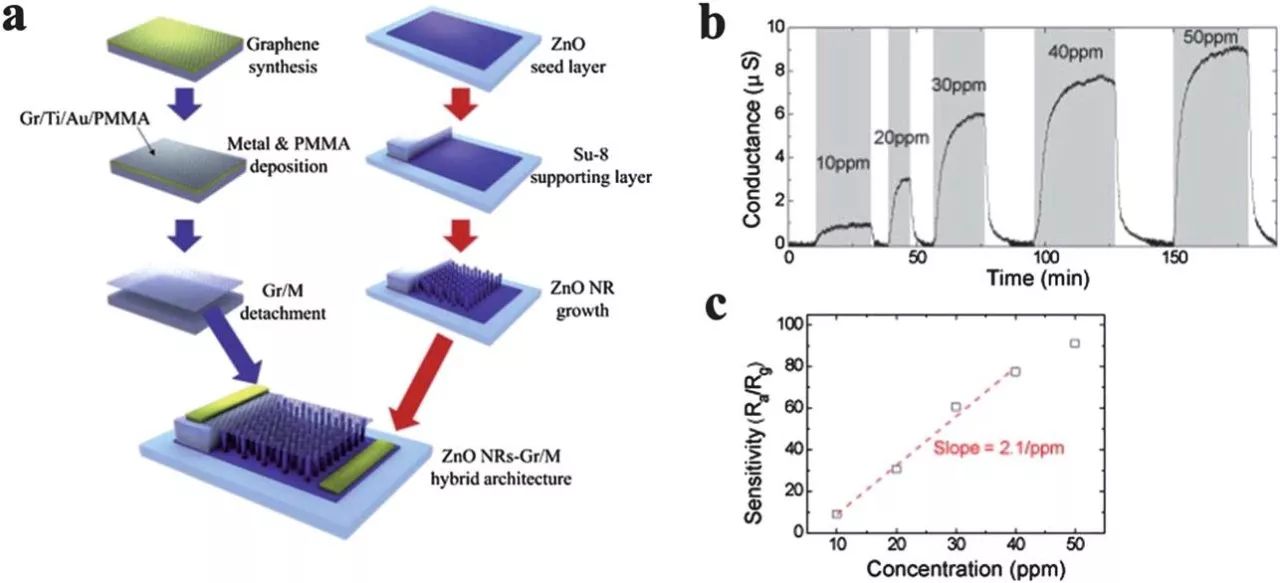
The RGO sheets of the crystals in the immobilized cuprous oxide nanowires were developed and the detection of nitrogen dioxide in gas sensor applications was developed. The amorphous is made of highly anisotropic nanowires and has a unique octahedral morphology. The response (Ig/I0 ≤ 1, where Igand I0 is, currently at the target gas and N 2 respectively) is 67.8% for the Cu2O amorphous/RGO hybrid material, 2 ppm NO 2, than RGO (22.5%) or more. High cuprous oxide nanowires (44.5%) are alone. The theoretically calculated LOD is 81 ppb with a composite of 64 ppb and 82 ppb for RGO and cuprous oxide, respectively.
The concentration in the mixed material that is significantly improved in detection performance is higher than 1.2 ppm. This phenomenon can be explained as follows. Metal oxides need to activate their oxygen ions to create an electron depletion layer on the surface. Diffusion on the active surface of gas molecules is a decisive factor for detecting high concentrations. Since RGO does not require oxygen activation, this factor is eliminated after mixing cuprous oxide with amorphous RGO. CNT is another one-dimensional structure suitable for the detection of various gas samples. However, the integration of CNTs into flexible substrates is a major problem due to poor contact with metal electrodes. The room temperature NO 2 gas sensor is based on the CNT / RGO hybrid film, and the vertically aligned carbon nanotube array is directly grown on the RGO film by effectively avoiding poor contact with the metal electrode by the CVD method. The resulting sensor exhibits a significantly enhanced sensitivity compared to the pure graphene.121 mass of the graphite-based graphene structure of the nanoparticles, which is an effective method for preventing agglomeration of graphene sheets during drying. Moreover, these nanoparticles can also provide composites with new physical and chemical properties and improve graphene-based sensitivity and selectivity sensors.
RGO/SnO2 and CNT/SnO2 nanoparticle (nano) composites have been widely used as ammonia detection and NO2 sensing materials. As a result, when the composite is exposed to NO 2 , more electrons are attracted to the SnO 2 from RGO, giving a greater conductivity increase than pure RGO. On the other hand, when it is exposed to NH 3 , fewer electrons are injected to change its conductivity compared to pure RGO. In order to further increase the selectivity, indium is introduced into the doped SnO 2 nanocrystals. On this basis, the composite sensor of graphene shows excellent selectivity for detecting NO 2 compared with NH 3 , H 2 , CO and H 2 S. 52 It also indicates that UV light irradiation is used when RGO/SnO 2 composite acceleration is used. The recovery rate of the sensing device exceeds the RGO-based device by a sequential magnitude. 122 UV light activated the reaction on the surface of the SnO 2 nanoparticles and developed a barrier to the heterojunction between the RGO sheet and the nanoparticles, resulting in a loss of conductance in the accelerated sensing device. However, if the density of the SnO 2 particles is increased to the value of the percolation threshold, the n-type response behavior of the tin dioxide becomes another electrical path, and thus the sensitivity and recovery are deteriorated.
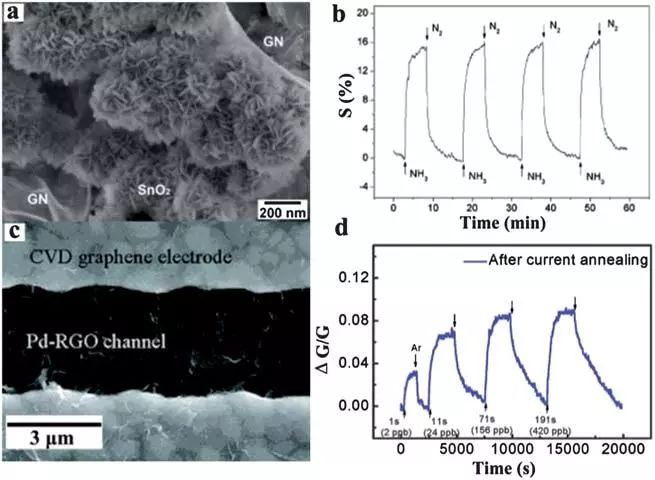
Dispersive metal gas sensors are generally very effective, but they are expensive. This problem can be bypassed by using a large SPECIC surface area deposited on other materials, such as graphene nanoscale particles or films. Precious metal decorative graphene nano hybrids are expected to have new sensing materials with high sensitivity and selectivity. For example, the H2 detection behavior of graphene sheets decorated with platinum nanoparticles (platinum nanoparticles) or Pt films has been studied. Similar to metal oxide based sensors, the role of Pt is to separate H2, which causes the H atoms to subsequently diffuse into the graphene sheets. The platinum NP/graphene composite sensor showed 4% by volume of (RG / ra ≤ 1) H 216% based on the high response of Pt / CNT (8%), twice the sensor. In another method, a gas sensor has been developed which is based on the preparation of an alternating current electrophoresis (AC-DEP) Pd/graphene composite for the detection of NO. This sensor has palladium-decorated RGO sensitive channels and electrodes that are covered with CVD graphene (Fig. 10C), and it is capable of detecting NO concentrations ranging from 2 to 420 ppb in response times over a few seconds at room temperature. In order to shorten the recovery time of the sensor, the Pd-RGO and the sensing signal which are further lowered with a medium current of 1 mA can be restored to their initial state after the current processing. The incorporation of metal nanoparticles into RGO also increases the selectivity of graphene-based gas sensors. For example, one sensor was fabricated using RGO/silver nanoparticle composites as sensing materials and showed higher or lower sensitivity to NH3 or NO2 compared to pure RGO, improving its selectivity to NH3.
The future and prospects of graphene gas sensorsGraphene materials and their composites are unique and attractive sensing materials used in the manufacture of sensors to detect toxic, flammable or explosive gases and typical equipment.
Compared to conventional metals, graphene-based sensors exhibit improved performance, oxide-based sensor sensitivity, reversibility, and detection limits. Moreover, such sensors can typically operate at low energy consumption at room temperature. High mechanical strength, large specific surface area and excellent temperature or electrical tolerance of graphene materials make them promising for candidate gas detection under adverse conditions. In addition, most gas sensing applications do not require high quality graphene sheets because the defective sites are generally beneficial for gas adsorption. For this purpose, the chemical reduction of GO is a priority route, resulting in a large number of defective graphene at low cost.
Despite this, there are still several issues that need to be addressed for commercial graphene-based gas sensors.
First, the selective detection of specific gases in gas mixtures has rarely been studied and the selectivity of these sensors is required to be improved. The sensing mechanism of most graphene-based sensors is the physical or chemical adsorption of the target gas. Various gas molecules can be adsorbed on the graphene sensing layer to give similar changes in conductivity.
Second, the manufacture of high performance graphene-based gas sensors should effectively avoid the effects of pollution and environmental interference. Conventional etching techniques can leave uncontrollable contaminants on the surface of the graphene sensor. Although these residues must have an active role in sensing performance, their contribution is uncontrollable. In addition, some air components such as moisture and water can also adsorb on the surface of the graphene of the material. After exposure to a target gas such as NO 2 or NH 3 , the adsorbed water molecules interact with these gases, making the sensing process more complicated and the sensing results unpredictable.
Third, manufacturing techniques suitable for ultra-thin, high-quality sensing layers are required. It is also difficult to manufacture ultra-thin graphene films by widely used techniques such as drop casting, spin coating, and ink jet printing. In addition, it is also the number of graphene sheets that are difficult to control.
Fourth, graphene and its patterns in remote sensing applications have not been extensively studied. Circuitry and complex designs can be patterned into substrates with photomasks or by laser scribing. All organic flexible electronics can be obtained in a single step process.
After solving the above problems and fully understanding the properties of graphene materials and their detection mechanisms, graphene-based gas sensors will have a bright and successful future.
New Smart Watch
New Smart Watch
everyone enjoys luck , https://www.eeluck.com